Latest News
- January 08, 2026Official press release: CBCC Global Research is now REV Clinical
- November 18, 2025ANDA application for Bimatoprost ophthalmic solution received Marketing Authorization from the USFDA!
- November 12, 2025ANDA application for Risperidone extended-release injectable suspension has received Marketing Authorization from the USFDA!
- October 30, 2025Accelerating Clinical Research!
- September 12, 2025Join CBCC at CPHI Frankfurt 2025
June 02, 2025
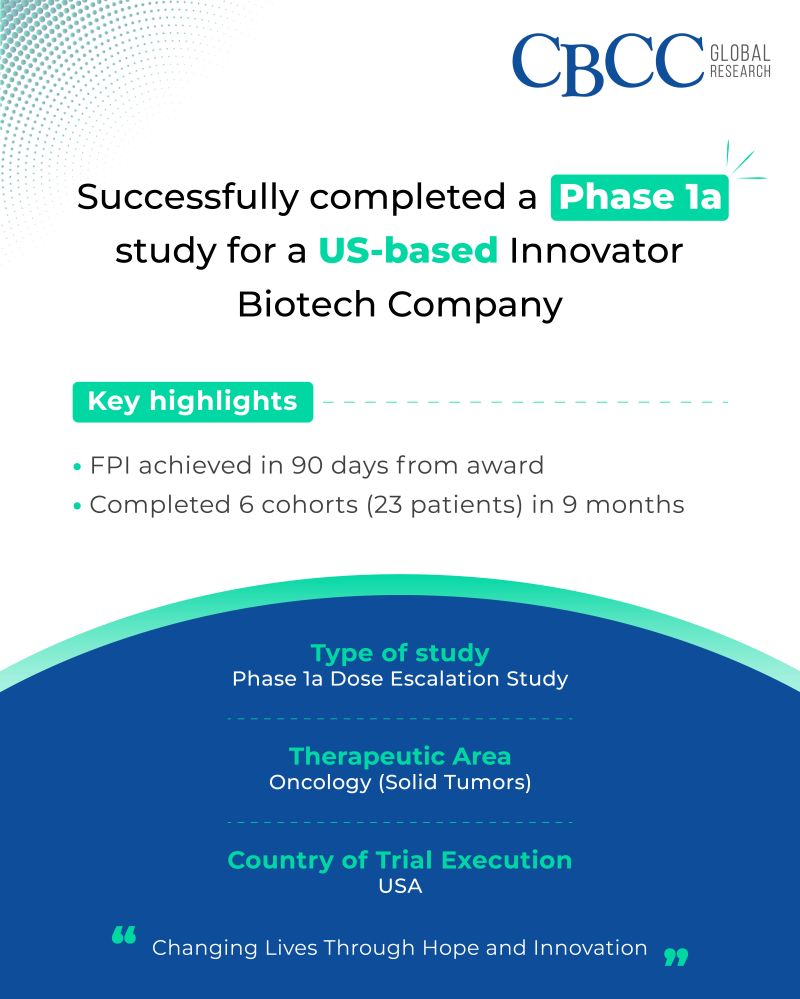
Successfully completed a Phase 1a study for a US-based Innovator Biotech Company!
 Successfully completed a Phase 1a study for a US-based Innovator Biotech Company!
Key highlights
FPI achieved in 90 days from award
Completed 6 cohorts (23 patients) in 9 months
Type of study: Phase 1a Dose Escalation Study
Therapeutic Area: Oncology (Solid Tumors)
Country of Trial Execution: USA
CBCC Global Research: CHANGING LIVES THROUGH HOPE AND INNOVATION
Manoj Vyas
Dr. Sandeep Singh
Jaimeen Vanparia
Sanjeev Ganatra
Mark Kirschbaum
Nikhil Gandhi
Harakh Shah, PMP®
Tapan Shah
Kinnari Gandhi RQAP-GCP
Dr. Praveen Choudhary
Nikunj Patel
Ankit Parikh
Ajit Upadhyaya
Ankit Pandav
Devang Pandya
Jay Vyas
Udayan Vyas
hashtagASCO25 hashtagOncologyResearch hashtagClinicalTrials hashtagCBCCGlobalResearch
hashtagInnovationInHealthcare
Successfully completed a Phase 1a study for a US-based Innovator Biotech Company!
Key highlights
FPI achieved in 90 days from award
Completed 6 cohorts (23 patients) in 9 months
Type of study: Phase 1a Dose Escalation Study
Therapeutic Area: Oncology (Solid Tumors)
Country of Trial Execution: USA
CBCC Global Research: CHANGING LIVES THROUGH HOPE AND INNOVATION
Manoj Vyas
Dr. Sandeep Singh
Jaimeen Vanparia
Sanjeev Ganatra
Mark Kirschbaum
Nikhil Gandhi
Harakh Shah, PMP®
Tapan Shah
Kinnari Gandhi RQAP-GCP
Dr. Praveen Choudhary
Nikunj Patel
Ankit Parikh
Ajit Upadhyaya
Ankit Pandav
Devang Pandya
Jay Vyas
Udayan Vyas
hashtagASCO25 hashtagOncologyResearch hashtagClinicalTrials hashtagCBCCGlobalResearch
hashtagInnovationInHealthcare
