Latest News
- January 08, 2026Official press release: CBCC Global Research is now REV Clinical
- November 18, 2025ANDA application for Bimatoprost ophthalmic solution received Marketing Authorization from the USFDA!
- November 12, 2025ANDA application for Risperidone extended-release injectable suspension has received Marketing Authorization from the USFDA!
- October 30, 2025Accelerating Clinical Research!
- September 12, 2025Join CBCC at CPHI Frankfurt 2025
February 19, 2025
Actinic Keratosis drug receives FDA Approval for which CBCC had conducted the Clinical Endpoint study in the US!
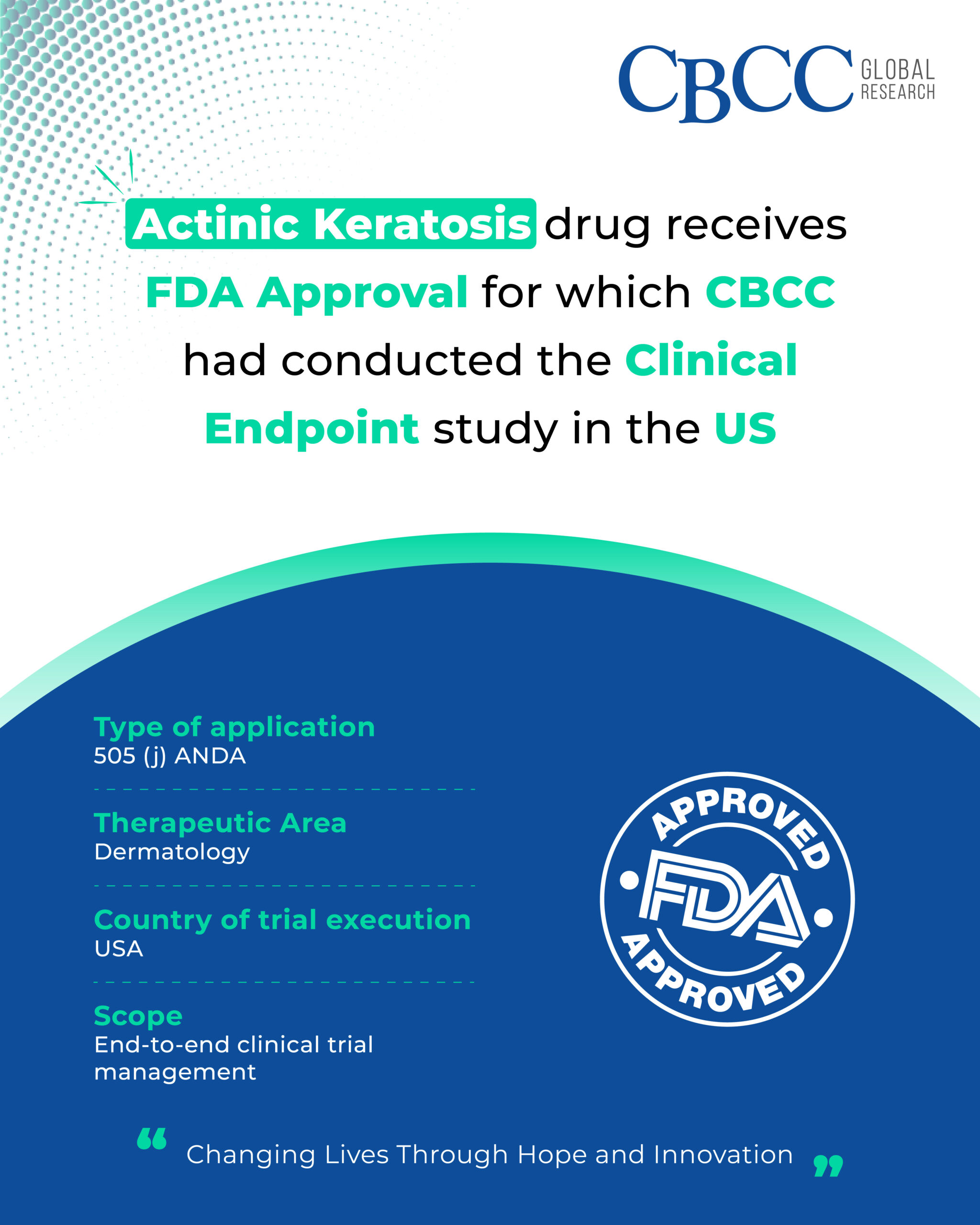 Actinic Keratosis drug receives FDA Approval for which CBCC had conducted the Clinical Endpoint study in the US!
Type of application: 505 (j) ANDA
Therapeutic Area: Dermatology
Scope: End-to-end clinical trial management
Country of trial execution: USA
CBCC Global Research: CHANGING LIVES THROUGH HOPE AND INNOVATION
Manoj Vyas
Dr. Sandeep Singh
Jaimeen Vanparia
Avanish Mishra, Ph.D.
Sanjeev Ganatra
Harakh Shah, PMP®
Nikhil Gandhi
Tapan Shah
Kinnari Gandhi RQAP-GCP
Dr. Praveen Choudhary
Nikunj Patel
Ankit Parikh
Ajit Upadhyaya
Ankit Pandav
Devang Pandya
Jay Vyas
Udayan Vyas
Actinic Keratosis drug receives FDA Approval for which CBCC had conducted the Clinical Endpoint study in the US!
Type of application: 505 (j) ANDA
Therapeutic Area: Dermatology
Scope: End-to-end clinical trial management
Country of trial execution: USA
CBCC Global Research: CHANGING LIVES THROUGH HOPE AND INNOVATION
Manoj Vyas
Dr. Sandeep Singh
Jaimeen Vanparia
Avanish Mishra, Ph.D.
Sanjeev Ganatra
Harakh Shah, PMP®
Nikhil Gandhi
Tapan Shah
Kinnari Gandhi RQAP-GCP
Dr. Praveen Choudhary
Nikunj Patel
Ankit Parikh
Ajit Upadhyaya
Ankit Pandav
Devang Pandya
Jay Vyas
Udayan Vyas
